 |
| Laws Of Thermodynamics In HVAC Design & HVAC system |
We in the weekend spend most of our time with friends, family and relatives to spend luxurious and sweet time with our lovings. But friends, when there is a rise of discomfort, one may not stand with each other. so as an Engineer we must make the world comfortable to live, which can be done by the study of Laws Of Thermodynamics In HVAC Design & HVAC system.
Now let’s focus on what are the basic Laws Of Thermodynamics In HVAC Design & HVAC system
Basically to design a better system or make a space comfortable we need to study the laws of thermodynamics which ultimately helps us to contribute towards the best design of the HVAC system.
Thermodynamic Definition in HVAC
Thermodynamics in HVAC is a branch of engineering science which deals with the study of heat and its forms of energy.
Thermodynamics is a field in which people plays with the temperature, to maintain desired condition suitable to a human being.
It plays a very important role in decorating this HVAC design by calculating the space with thermodynamics derived formulas.
Before moving to thermodynamics laws we need to understand the basic thermodynamics terms
- HEAT
- TEMPERATURE
- BRITISH THERMAL UNIT
- ENTHALPY
- SENSIBLE HEAT AND LATENT HEAT
WHAT IS HEAT 😕?
Heat is basically a form of energy which transfer due to change in temperature
Unit:KJ and BTU
WHAT IS TEMPERATURE😕?
Temperature is basically a degree of hotness and coldnessUnits: °F and °c

So let's clear this confusion so that we can move the topic smoothly without a piece of confusion in further learning.
Basically, heat and temperature are two different terms, which we will understand with an example, let's take a container with water and put it on cooking gas for some time, you will find the water gets heated, after some time.
So heat generated by cooking gas is an energy which is been absorbed by the water through the container and to measure the heated water we have a term called temperature which is a scale to detects it the degree of hotness or coldness depending on the conditions. Hope we are clear with temperature and heat.
In short, heat is the energy supplied to an object which is measured in terms of temperature.
Now understand the units of temperature and heat!
HEAT is basically measured in either kilojoule or British thermal unit
we define it!
Kilojoule (SI UNITS): it is defined as the amount of heat which need to be added to raise the temperature of 1kg of water by 1° C.
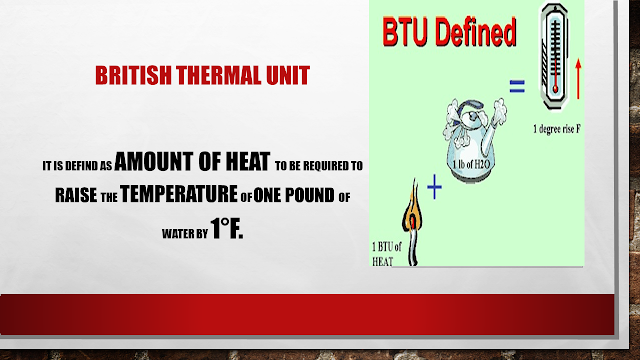
British Thermal Unit(BTU) (ENGLISH OR IMPERIAL UNITS): it is defined as the amount of heat which is been added to raise the temperature of 1 pound of water by 1°F.
So let us discuss this what actually it is ………..
Basically, let us take the same example of a container with water on cooking gas, this units of energy define the amount of heat energy required to heat 1 kg or 1 pound of water by 1° C or 1°F. which indicated the capacity of heat energy required to obtain the desired heating temperature.
ENTHALPY
Enthalpy is basically the amount of heat energy contained in a body in short.
Now let's define it, so basically it is defined as the sum of internal energy and the product of pressure and volume.
ENTHALPY Definition
H = µ + PVµ= Specific heatp=pressurev=volumeUnit KJ/HR OR BTU/HR
Basically, in short, we can define it as the rate of heat absorbed or heat release by a system or from the system per unit time.
Sensible and Latent Heat
Sensible and Latent heat is a very important parameter to discussed, as it plays a vital role in HVAC Design and HVAC System.SENSIBLE HEAT
Sensible heat is the heat which is sensible this answer is something you will not expect but it is true. sensible heat is a heat which is sensible or measurable.
LATENT HEAT
Latent heat is the heat which is not measurable but the heat is utilized to change the phase either from solid to liquid or liquid to gases.
 |
Laws of thermodynamics in HVAC
Basically, there are certain laws, which is been added in this thermodynamics after studies and there are four law
- Zeroth law of thermodynamics
- The first law of thermodynamics
- Second laws of thermodynamics
- Third laws of thermodynamics
Basically out of this we will focus on the first three laws which rotate around HVAC designing.
 |
| ZEROTH LAW OF THERMODYNAMICS |
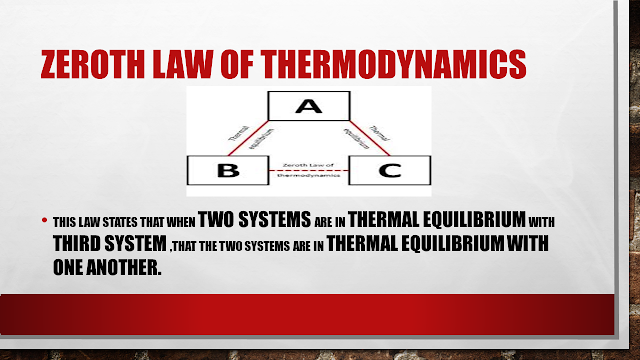
James Clerk Maxwell was who put forward the law which states that when two body is in thermal equilibrium with the third system than two systems are in thermal equilibrium with one another.
Basically, it says that consider there are there system A, B AND C B AND C have the same thermal temperature with A that means B and C also have the same temperature.
This question must be arriving in your mind why it is called zeroth law it could also be called as First law ????
Well, it was discovered after the discovery of first law hence it is named as Zeroth law.
FIRST LAW OF THERMODYNAMICS
the first law of thermodynamics is a version of the law of conservation of energy, adapted for thermodynamic processes, distinguishing two kinds of transfer of energy, as heat and as thermodynamic work, and relating them to a function of a body's state, called Internal energy.
The law of conservation of energy states that the total energy of an isolated system is constant; energy can be transformed from one form to another but can be neither created nor destroyed.
Well, in short, it says that heat and work are mutually convertible
Consider a system like a bicycle in which paddling is heat energy and movement of the bicycle is mechanical work. Now if the person is running that means he is doing mechanical work which is been converted into the release of energy in terms of calories burned. Hence heat energy can be converted into mechanical work and vice versa
 |
| The second law of thermodynamics |
Basically, there are two statements in it
- Clausius statement
- Kelvin plank statement
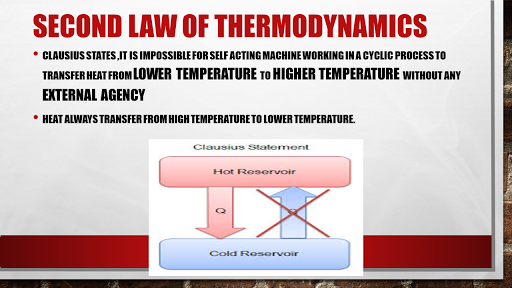
But we only need to deal with Clausius statement which states that it is impossible for self-acting machine working in a cyclic process to transfer heat from a body at a lower temperature to higher temperature without any external agency.
Before understanding this we should be clear that heat always flows from higher temperature to lower temperature.
Well now let us understand the law, so it meant to say that there is "no, any machine which transfers heat from lower temperature to higher temperature".
If we want it to happen we cannot do it without any external agency which maybe some electrical and mechanical machine.
Hope we are clear with it !!!!!
NOW MOVE ON TO ........
MODES OF HEAT TRANSFER
Well now we know what is heat transfer and it flows from higher to lower temperature. But how does it flows ???????
Basically, it flows through mediums which are as follows:
- Conduction
- Convection
- Radiation
This is a medium through which heat transfer, let us understand it in details
 |
| Conduction |
What Is Conduction
CONDUCTION: well it is a heat transfer process which generally takes place in solid bodies with physical movement.
Well to understand it lets take an example which we do it daily that takes iron rod which has good conductivity to heat and start heating it from one end, ultimately the rod on another site too get heated after some time.
Well, how does it transfer heat 😕??????
conduction takes place in solid bodies in which the molecules are very closed to each other having strong bonds.
When heated at one side of the rod the strongly bonded molecules started vibrating due to heat absorption and which ultimately pass the vibration to others which generate frictional heat too.
Hence these vibrations are been transferred to every molecule which generates friction due to motion and passes the heat.
 |
| What Is Convection |
What is convection
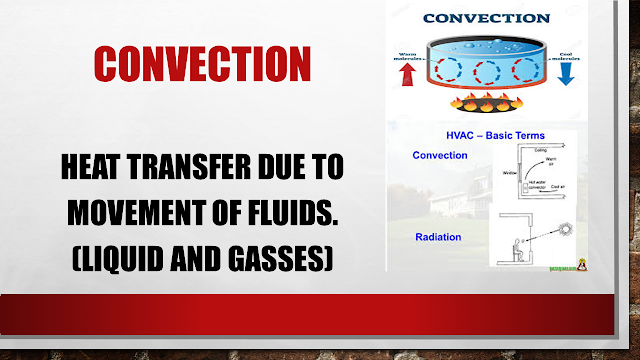
CONVECTION: It is a heat transfer process which generally takes place in liquid bodies or gases bodies due to its movement due to heat absorption.
So to understand it lets say water in the container is heated on cooking gas, so the heat is transfer through container via conduction and after the convection start where it absorbs the heat and transfer within the water throughout.
Well, how does it transfer heat??????
Basically, convection takes place in liquid bodies in which the molecules are very less closed to each other having less strong bonds than solid.
Heated water absorbs the heat which is closed to container and as it takes the heat its density reduces and hence it gets on top or rim of the container while denser water comes close to the heated container.
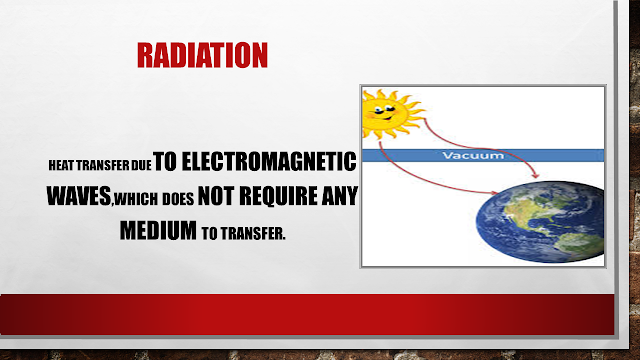 |
| What Is Radition |
What is Radiation
RADIATION: This is the medium which doesn’t require any medium to transfer heat. It can transfer the heat even in a vacuum.
How does it transfer😕????
Basically, this is very deep but it transfers through electromagnetic waves.
This is all about HEAT TRANSFER .................
Hope we are cleared with it this term. These terms are very important and foundation of HVAC which will ultimately help us to gain profitable design of the desired space.
This is all about Laws Of Thermodynamics Used In HVAC Design & HVAC system .Hope you like it and share with your Lovings and follow us on the given link...
For More Information Follow Us On :
Facebook link: https://bit.ly/38xl2Po
Pinterest Link: https://bit.ly/2BKEegD
Instagram Link: https://bit.ly/3fbAyD6
LinkedIn Link: https://bit.ly/3gA30ih
Twitter Link: https://bit.ly/38zmTmN






0 Comments